 The forces driving tissue loss after cerebral ischemia are temporally and mechanistically diverse involving cell-autonomous and non-autonomous responses. Necrosis, regulated cell death (RCD), and neuroinflammation cause tissue damage and impede functional recovery. Also, the shedding of damage-associated molecules heightens peripheral immune activation and reperfusion injury. This complexity remains a fundamental challenge to developing effective neuroprotective strategies. In 2017, we launched a program to identify neuroprotective substituted tetracyclines for use in cardiac arrest and stroke. Supported by an award from the Department of Defense (PR150522), we have thus far developed and tested over 75 novel tetracycline derivatives in collaboration our partners at Echelon Biosciences (Salt Lake City, UT).
The forces driving tissue loss after cerebral ischemia are temporally and mechanistically diverse involving cell-autonomous and non-autonomous responses. Necrosis, regulated cell death (RCD), and neuroinflammation cause tissue damage and impede functional recovery. Also, the shedding of damage-associated molecules heightens peripheral immune activation and reperfusion injury. This complexity remains a fundamental challenge to developing effective neuroprotective strategies. In 2017, we launched a program to identify neuroprotective substituted tetracyclines for use in cardiac arrest and stroke. Supported by an award from the Department of Defense (PR150522), we have thus far developed and tested over 75 novel tetracycline derivatives in collaboration our partners at Echelon Biosciences (Salt Lake City, UT).
Neurons also exhibit remarkable structural and functional specialization required for their unique properties including excitability and synaptic plasticity. This diversity is mirrored in the neuronal proteome through the exquisite temporal and spatial regulation of transcription factors, kinases, phosphatases, and other effector proteins. However, at present there a total of 1,194 small molecules and biologics that target 667 proteins approved by the FDA for use in humans. And while the tenet “the best way to discover a new drug is to start with an old one” has been an effective profit strategy for the pharmaceutical industry, it has created a bottleneck in the pipeline of novel targets and therapies for stroke and CNS disorders. The NeuroTag project will test the hypothesis that location proteomics will expedite the identification of druggable targets most relevant to cell-autonomous death signaling pathways activated by CNS ischemia.
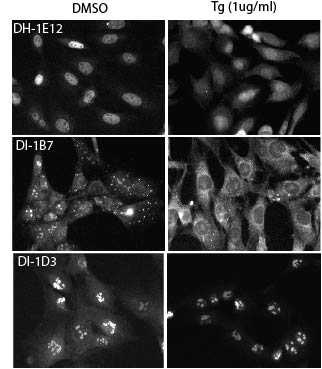
Halterman MW. An improved method for the study of apoptosis-related genes using the Tet-On system. (2011) J Biomol Screen. 16(3):332-7. PMID: 21335597.
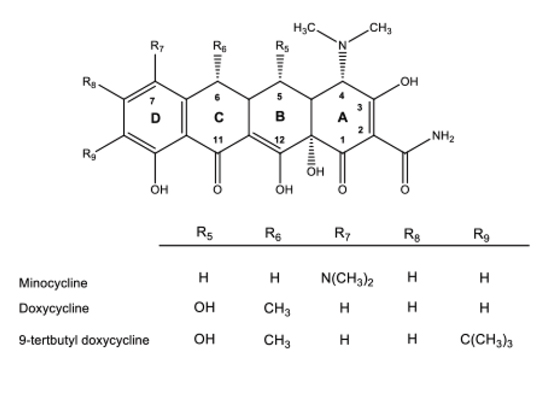
Knowlden SA, Mai N, Miller-Rhodes K, Prifti V, Sims M, Grier M, Nelson ML, and MW Halterman. (2019) Improved CNS delivery and effects of 9-tert-bytyl doxycycline on neutrophil polarization following cerebral ischemia-reperfusion injury. ACS Infectious Disease.