Stony Brook University Hospital Antibiograms (updated 2/20/25)
Links to University Hospital's 2025 Antibiograms are below:
Stony Brook University Hospital Antibiogram (2025 data, COMMUNITY origin isolates)
Stony Brook University Hospital Antibiogram (2025 data, HOSPITAL origin isolates)
Antibiogram Notes
In general, cultures obtained from outpatients are more likely to have drug susceptible bacteria compared to cultures obtained from inpatients. The Community antibiogram is more likely to reflect those patients seen in our clinics and those patients seen in our emergency room without recent healthcare exposures (i.e. hospitalization within the past 30 days). This data may be more applicable to those less sick patients without risk factors for multidrug resistant pathogens.
For patients at risk for nosocomial infections (occurring >3 days in the hospital), the Hospital based antibiogram should be used to help guide antibiotic therapy as it may better reflect the resistance patterns encountered.
The CLSI M100 reference standard is used for antibiotic breakpoints. Based on the most recent update, some bacteria are now considered to be intrinsically resistant to antibiotics that have been previously used for treatment. Those antibiotics for which a bacteria is considered to be intrinsically resistant are now labelled with a "R".
8/12/25
Not all diarrhea is Clostridioides difficile! Clostridioides difficile Testing Criteria
Diarrhea is a common complaint in the hospital. Before you send a C.difficile PCR, refer to the testing criteria below:
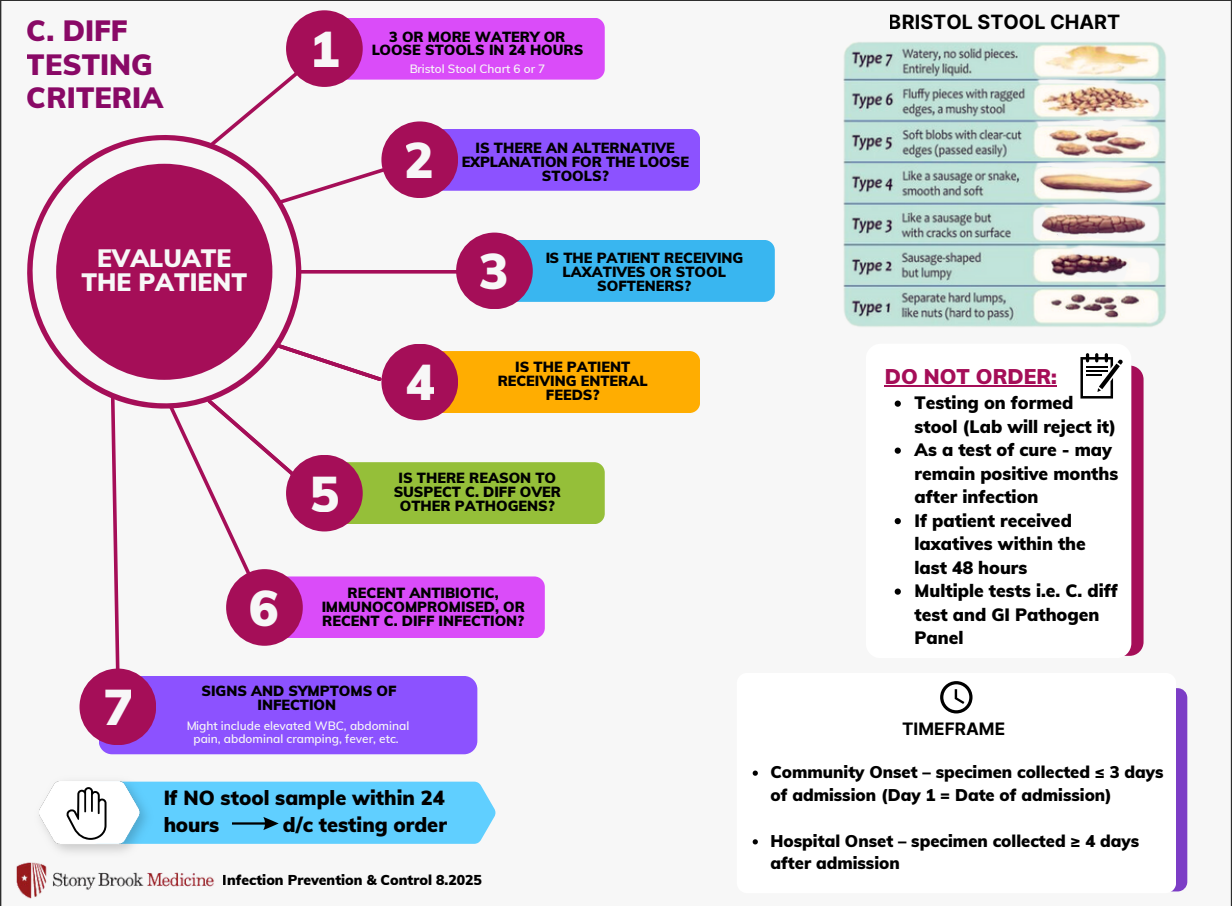
05/5/25
Pharmacy Driven IV to PO Conversion (SBM Policy MM0092)
Intravenous (IV) to oral (PO) therapy interchange programs are often used in hospital settings to promote cost-effective utilization of medications. Research shows that appropriately transitioning from IV to PO antimicrobial therapy can shorten hospital stays without compromising patient outcomes. Moreover, this approach may enhance patient care by reducing the risk of catheter related infections and offering advantages such as increased comfort, reduced nursing demands, and improved mobility. As patients progress and prepare for discharge, transitioning from parenteral to oral therapy becomes a viable option, provided the medication achieves the required concentrations in the bloodstream or targeted areas. The conversion from IV to PO formulations of the same medication while maintaining equivalent potency is known as “sequential therapy”
Pharmacists will use predetermined criteria to screen for patients who may be eligible for IV to PO conversion for certain antibiotics. If your patient qualifies, they will notify you of the change.
The medications and protocols are site specific. Please refer to the following links:
11/19/24
Updated Institutional Treatment Guidelines
Stony Brook Medicine Clostridioides difficile Guidelines (Updated 5/5/25)
Stony Brook Medicine Neutropenic Fever Treatment Guidelines
Stony Brook Medicine Invasive Fungal Infection Treatment Guidelines
Stony Brook Medicine Community Acquired Bacterial Pneumonia Treatment Guidelines (updated 4/16/2024)
Stony Brook Medicine Community Acquired UTI Treatment Guidelines
Stony Brook Medicine COVID Treatment Guidelines (updated 11-19-24)
11/18/24
Upcoming Changes to Antibiotic Restriction Policy (MM0086)
Changes are coming to Stony Brook Medicine's Antibiotic Restriction Policy (MM0086). Moving forward, restrictions will be separated into two tiers:
- Tier One: Requires Infectious Diseases consultation within 24 hours for continued use
- Tier Two: Requires either Infectious Diseases or Antimicrobial Stewardship Program approval for use
More information regarding implementation of this policy will be forthcoming.
Restricted Antibiotic Standard Operating Procedure
8/28/24
Blood culture Guidelines. When and how many?
Blood cultures are a staple test when evaluating patients with infections. But does everyone with an infection need blood cultures? The decision to order blood cultures should depend on whether a patient has a new diagnosis of sepsis or if there is high concern for bacteremia. The Diagnostic Stewardship working group has written guidance on when blood cultures may be indicated as well as how many and how often the test should be done.
Note: due to the nationwide shortage of BD Bactec blood culture vials, restrictions on the ordering of blood cultures may be imposed that are not in accordance with these guidelines.
Blood Culture Guideline for Non-Severely Immunocompromised Adult Patients
Blood Culture Guideline for Immunocompromised Patients without Neutropenic Fever
10/31/2024
Formulary updates
Stony Brook University Hospital has recently added the following antimicrobials to the formulary:
Sulbactam-durlobactam (Xacduro)
This beta lactam-beta lactamase combination has been approved for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia cased by susceptible strains of Acinetobacter baumannii-calcoaceticus complex. Acinetobacter infections can be challenging to treat in the hospital setting as it is common to encounter multidrug resistant strains. Sulbactam-durlobactam is not FDA approved for the treatment of causes of HAP/VAP other than Acinetobacter baumannii-calcoaceticus complex. Infectious Diseases consultation is required for use beyond 24 hours.
Antibiotic Shortages (updated 11/10/24)
- Linezolid IV
- IV Fluid shortage (indirectly impacts on intravenous antibiotics that need to be reconstituted for infusion)