The Okeoma laboratory investigates how host factors expressed in host cells or packaged into extracellular particles, such as extracellular vesicles (EVs) and membraneless condensates (MCs) protect the host against infective agents or facilitate disease manifestation. Our translational experiments use primary cells, animal models, and human clinical specimens to study spatiotemporal regulation of host factors and the effects on clinical outcomes. We use integrative scientific approaches including multi-modal datasets, computational modeling, Omics technologies along with experimental tools of cell and molecular biology in our studies. Discoveries made through this area of research will expand our knowledge and potentially lay the foundation for development of new tools, treatment, and prevention of disease. The Okeoma laboratory and Stony Brook University provide an environment that encourages, nurtures, and values diversity, inclusivity, and innovation. Outlined below is the main focus of our research laboratory.
Extracellular vesicles (EVs) in host:virus interactions: We perform basic and translational studies using specimens from HIV-infected human participants and SIV-infected rhesus macaque model of HIV/AIDS to:
- Investigate EVs and their spatiotemporal adaptations to the host environment, including viruses (HIV/SIV) and psychostimulants (cocaine and cannabinoids). We use Omics technology and meta-analyses of multi-modal large datasets to create EV compositional maps in time and space. We also correlate the compositional differences to host responses and disease outcomes, as well as identify interspecies differences and similarities to such responses.
- Investigate EVs as conduits by which drug-induced epigenetic mechanisms lead to biological changes that alter host response, promote microbial transmission, and disease progression. The major EVs that we study are blood and semen derived EVs. These body fluids serve as a conduits for transfer of infectious agents or immune factors to proximal and distal locations. In addition, semen is a major component in propagation of life―reproduction. Thus, we use experimental tools of cell and molecular biology to investigate how blood and seminal EVs disseminate factors that alter host responses – negatively or positively.
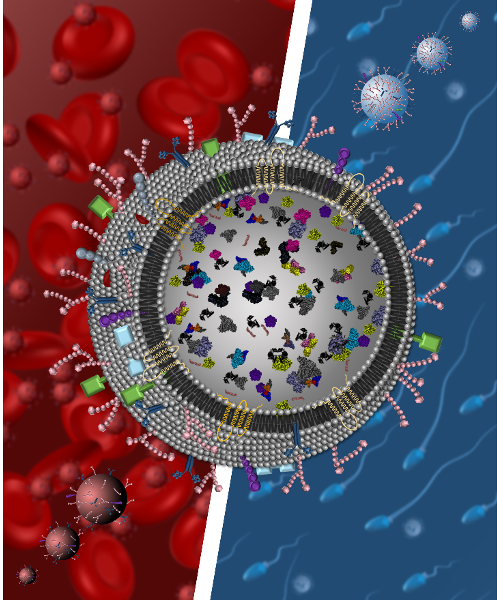
Blood and seminal EV
Identifying novel drivers of disease pathogenesis: In this area of research, we leverage tools in virology, cancer biology, and mouse genetics to identify novel drivers of disease pathogenesis. Our main focus is the restriction factor—Tetherin/BST2/CD317; which is a membrane-tethered glycoprotein expressed at basal levels on the surface of healthy cells. However, BST-2 expression is upregulated in virus-infected cells and in many cancer cells. The varied context and spatiotemporal expression of BST-2 makes it a protein with antiviral, proviral, and oncogenic functions. Thus, our focus is to:
- Use BST-2 as a model for identifying spatiotemporal drivers of molecular conflicts and mutually antagonistic relationships (antiviral, proviral, oncogenic functions). Our studies use BST-2 knock out mice and BST-2 knock out/PyMT transgenic mice, as well as various virus-infected and cancer cells.
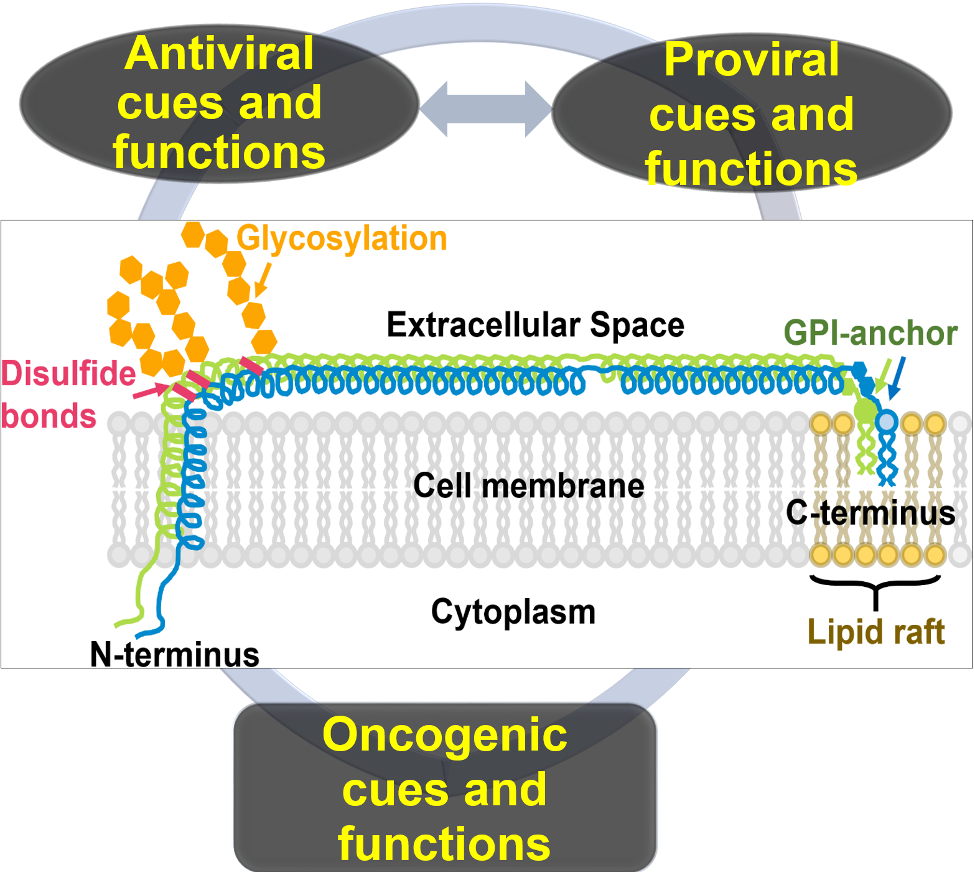
BST-2 in various contexts
Method and Resource development: To serve the research community, we are leveraging our technical expertise to develop resources for EV and cancer studies. To this end, we are:
- Developing Exomera as a Tandem analytical and preparative system (TAPS) for EV isolation and characterization. When developed, Exomera will be highly useful for EV sub-population isolation retrieval, and characterization. Unlike the current EV isolation tools, Exomera will be affordable and deployable to most laboratories. https://innovation.stonybrook.edu/content/exomera-extracellular-vesicles-purifier-and-analyzer
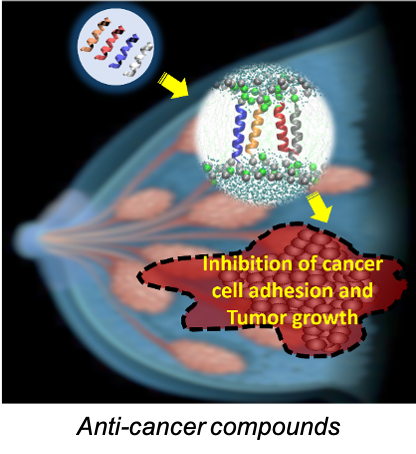
- Developing BST-2-based anti-cancer agents. We have developed the first in class BST-2-based anti-adhesion peptide series called B49 and B49Mod1. These peptides inhibit BST-2-mediated cell to cell and cell to extracellular matrix interactions. The peptides also inhibit tumor growth in the 4T1 mouse model of breast cancer. https://patents.google.com/patent/WO2017011375A1/en. Through structure activity relationship studies, we have identified the smallest fragment of the B49/B49Mod1 peptide series that retains the anti-adhesion property. This smaller peptide fragment is B18. Our current effort is focused on engineering a B18-based peptidomimetic as a targeted and specific cytotoxic agent against drug-resistant breast cancer.