 | Eckard Wimmer Professor Emeritus Department of Microbiology and Immunology Dr. rerum naturalium, University of Goettingen, Germany, 1962 | |
| E-mail: Office: Fax: | eckard.wimer@stonybrook.edu (631) 632-8787 (631) 632-8891 | |
| ||
| Honorary Societies and Awards | 2014 Loeffler-Frosch Medal by the Society of Virology of Germany, Austria and Switzerland 2012 Member, National Academy of Sciences 2012 Robert-Koch Medal in Gold by the Robert-Koch Stiftung, Berlin, Germany The 2010 Beijerinck Price in Virology, by The Royal Netherlands Academy of Arts and Sciences Fellow of the American Association for the Advancement of Science (2008) Lifetime Achievement Award, The Research Foundation of the State University of New York, April 2008 Distinguished Professor of the State University of New York at Stony Brook (2002) Fellow, Deutsche Akademie der Naturforscher Leopoldina von 1652 (1998) Fellow, American Academy for Microbiology (1994) Alexander von Humboldt-Forschungspreis (1996) | |
| Research | We are excited about viruses, viewing them either as chemical entities whose intriguing facility of proliferation is fascinating, or as horrifying infectious agents causing appalling diseases in their host. Accordingly, research in our laboratory can roughly be divided into two general topics: viral replication and genetics at the cellular level, and mechanisms of viral pathogenesis in a host organism. Our research focuses on RNA viruses, particularly picornaviruses, whose prototype is poliovirus. Picornaviruses, are estimated to infect 6 billion humans per year, cause a bewildering array of disease syndromes (paralysis, meningitis, heart disease, hepatitis, common cold, etc). 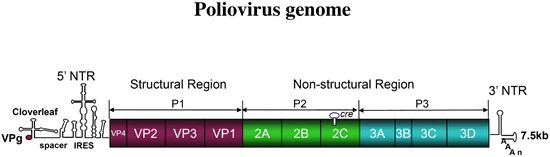 Paul, 2002 Picornaviruses contain a plus-stranded RNA genome that functions as mRNA as soon as the viral particle enters the cell. The viral proteins, which are synthesized, recruit cellular factors. Together, they provide a menu for genome replication and genome encapsidation. Topics studied in the laboratory are the control of translation independently of a capping group (mediated by the Internal Ribosomal Entry Site, IRES), RNA replication and viral genetics. We can reproduce the entire replication process in a cell-free extract.
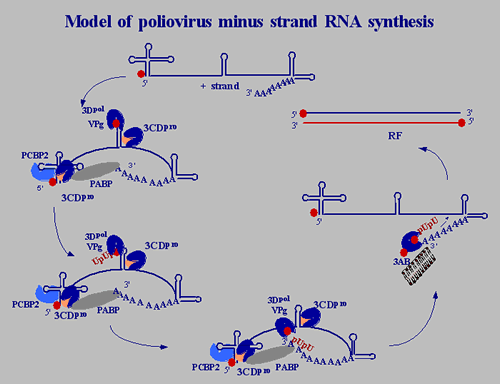
Molecular mechanisms by which a virus causes disease in a host organism are complex and multi level. We have focused on poliovirus, a virus known for nearly 100 years that in nature infects humans only but can also cause disease in primates and transgenic mice. Poliovirus replicates somewhere in the orogastrointestinal tract (Peyer's patches?) from which it can migrate to the central nervous system where it targets motor neurons. Destruction of motor neurons causes irreversible paralysis or death, a disease called poliomyelitis. The human receptor for poliovirus (CD155) is the key for pathogenesis as it is the gate for viral entry into cells. Therefore, the interaction of CD155 with poliovirus, its role in viral spread in the host organism, and its non-pathogenic cellular function(s) are all topics studied in the laboratory. In addition, we are investigating how genetic elements of the viral genome influence neurovirulence. Viruses are viewed to reside at the threshold between dead and living matter. This definition is flawed, however, because humans have not come to a consensus about the definition of life. When we chemically synthesized poliovirus according to its genomic sequence in the absence of natural template (Cello et al., 2002) we have shifted the definition of viruses towards non-living matter. Indeed, we view poliovirus as a chemical with a life cycle. As chemical it can be described with the empirical formula of its organic matter (Molla et al., 1991): C332,652 H492,388 N98,245 O131,196 P7501 S2340 Empirical formula of poliovirus Once inside the cell, however, poliovirus expresses the hallmarks of a living entity: multiplication, heredity and variation (John Maynard Smith, 1975), and even some form of primitive sex (recombination with siblings and closely related coxsackie viruses). Proliferation of poliovirus, the organism, is driven by an irresistible desire to maintain an optimal genome structure, thereby applying an inexhaustible arsenal for achieving this goal.
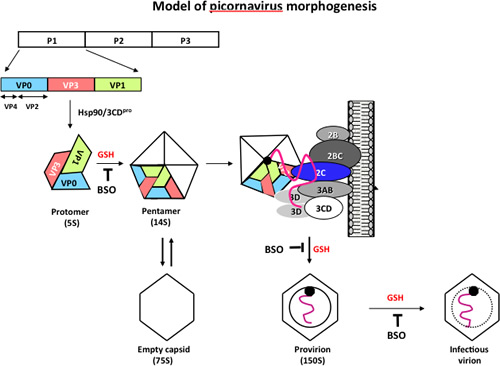 Our recent studies focus on a poorly understood step in the life cycle of poliovirus, the encapsidation of newly made viral RNAs. We have discovered that the specificity of encapsidation is determined by an interaction between nonstructural protein 2C and capsid proteins rather than on a RNA based signal, which is commonly used by RNA viruses. In addition, we have identified glutathione, a cellular reducing agent, as an essential factor for morphogenesis. | |