| Associate Professor Department of Microbiology and Immunology PhD, Stony Brook University, 1997 | |
| E-mail: Office: Fax: | nicholas.carpino@stonybrook.edu (631) 632-4610 (631) 632-9797 | |
| Publications | ||
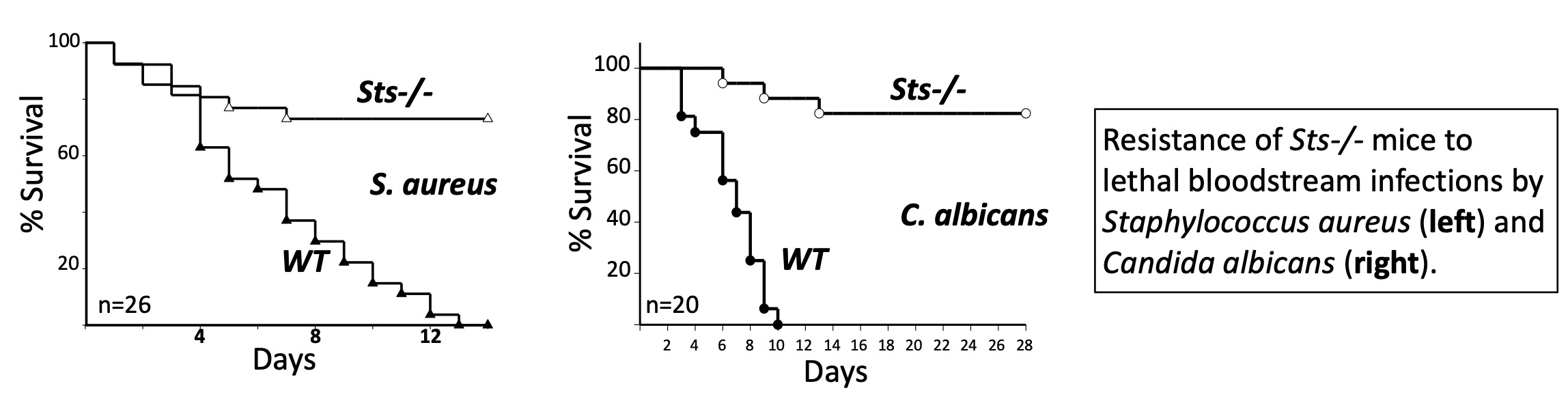
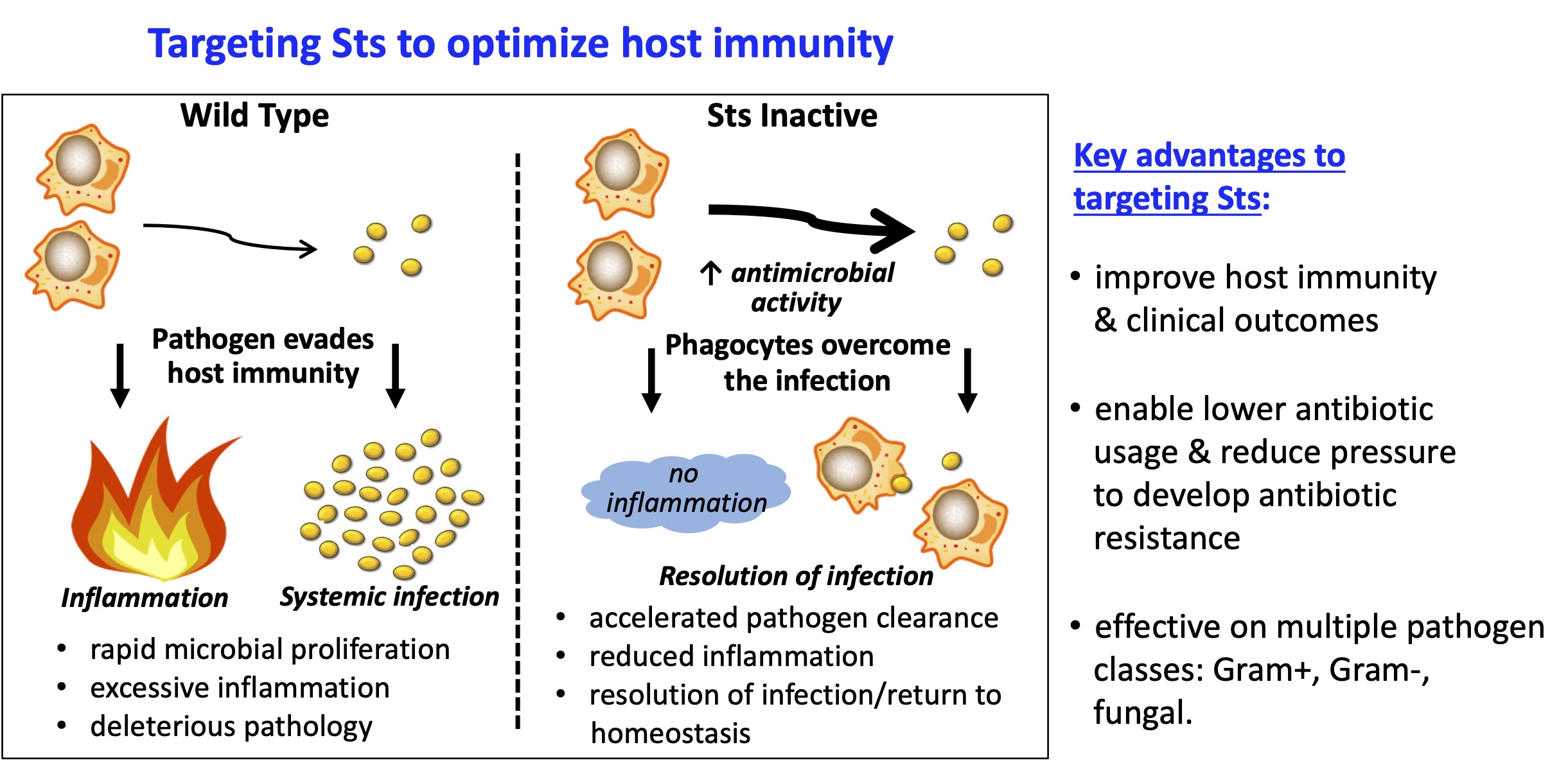
Research | Carpino Lab ProjectsOverview: Regulation of host responses to pathogen infectionWe focus broadly on host-pathogen interactions, the regulation of the mammalian immune response, and how inter-connected biochemical regulatory mechanisms control antimicrobial pathways. We study aspects of both innate and adaptive immunity, with an interest in understanding how the immune system balances the conflicting needs of eliminating dangerous microbes without simultaneously harming the host. Our research centers around two fascinating immunoregulatory enzymes known as Sts-1 and Sts-2, with studies anchored around two main goals: 1) elucidate biochemical, cellular, and in vivo mechanisms by which the Sts proteins regulate antimicrobial immunity; and 2) develop small molecule Sts inhibitors to advance research and drug development efforts. 1. Role of the Sts enzymes in modulating host immunityOur studies have implicated the Sts enzymes as critical antimicrobial immune checkpoints. Intriguingly, genetic inactivation of the Sts genes significantly increases host resistance to infection by diverse bacterial and fungal pathogens. To further understand the immunological basis of the Sts-/- resistance phenotype, this project focuses on establishing how the Sts proteins regulate host antimicrobial immunity.
Using several infection models, we are:
2. Deciphering the intracellular role of the Sts proteinsThe Sts enzymes are negative regulators of signaling pathways in a number of different hematopoietic cells, including T cells and several innate immune lineage phagocytic cell types. They have a unique, multi-domain structure consisting of two protein-protein interaction domains, a C-terminal HP (histidine phosphatase) domain and a region that contains motifs highly conserved among members of the 2H-phosphoesterase superfamily of enzymes. 2H-phosphoesterases are known to possess intrinsic phosphodiesterase (PDE) enzyme activity. This project focuses on investigating the intracellular mechanism(s) of action of the Sts proteins. We are:
3. Drug developmentDespite tremendous advances in the treatment of infectious diseases over the last century, a number of bacterial and fungal pathogens continue to pose grave health challenges. One problem is that many current antibiotics have shortcomings such as toxicity and limited bioavailability. We believe that developing treatment strategies that promote enhanced antimicrobial immunity can significantly improve clinical outcomes. This project will lay the groundwork for developing a first-in-class innate immune checkpoint inhibitor strategy to reduce lethality associated with select pathogenic microorganisms. Our strategy is based on the foundational observation that genetic inactivation of Sts leads to significantly reduced mortality following lethal inoculums. We have identified novel small molecule inhibitors of Sts and used these inhibitors to demonstrate that pharmacological inactivation of Sts is both achievable and phenocopies the antimicrobial resistance phenotype exhibited by Sts-/- mice. For this project, we are advancing hit compounds to the lead stage and determining the effects of acute Sts inhibition on host responses to infection.
| |