|
Research
|
The incidence of end-stage kidney disease is increasing worldwide and represents a major clinical and economic burden. Currently there are no effective treatments for this fatal condition. The kidney is an organ particularly susceptible to damage caused by infections and autoinflammatory conditions. Even so, renal immunology remains remarkably understudied by the immunologists. Over the past twelve years, the Biswas laboratory has successfully positioned itself to fulfill this important knowledge gap. Using mouse models and patients with kidney disease, the major goal of Biswas Laboratory is to understand the basic molecular and cellular mechanisms of kidney damage during autoinflammatory conditions and infections and utilize this knowledge for therapeutic benefit. Thus, research in the Biswas laboratory embodies the features of a basic scientist with scientific contributions spanning from very basic work in cell/animal models to more clinical work in humans.
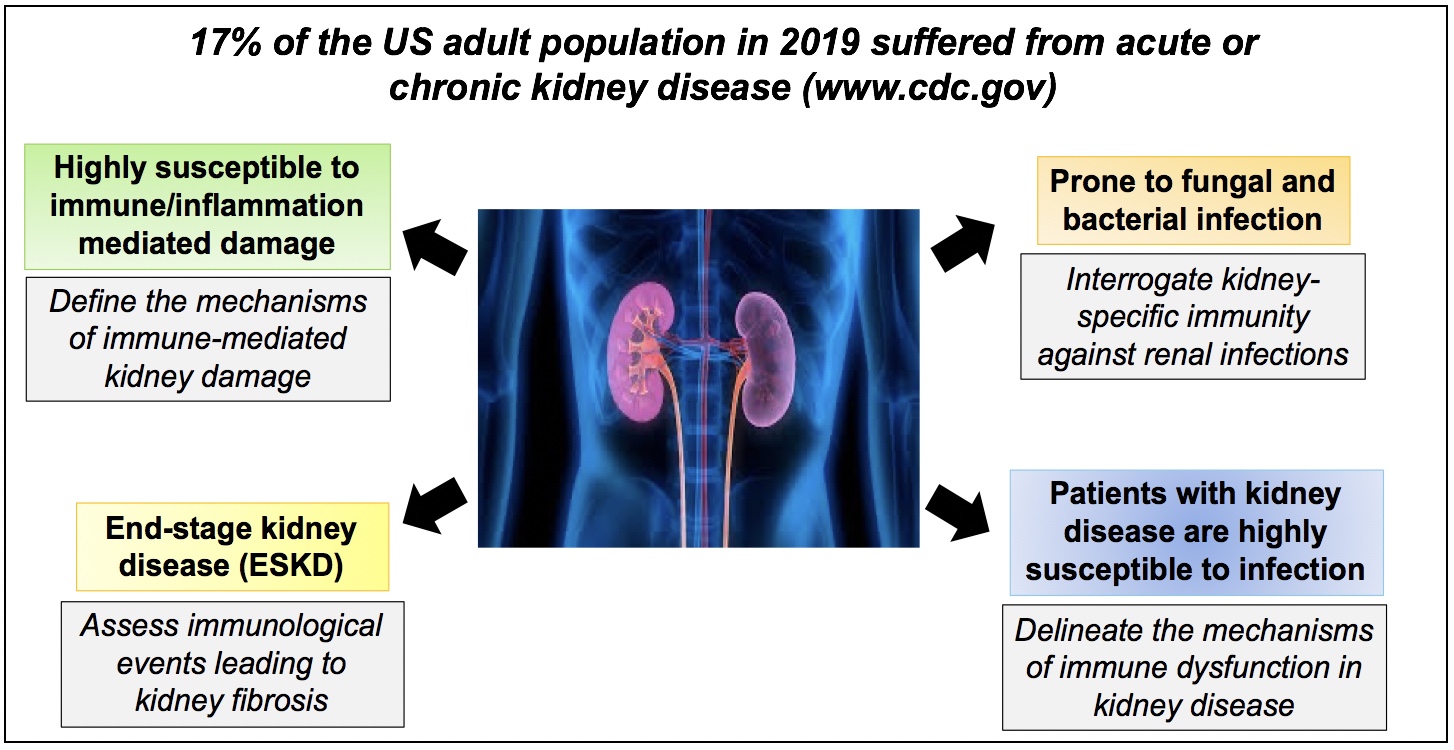 The research in the Biswas Laboratory is divided into several areas, centered around understanding “organ-specific” immunological events in the kidney during infection and autoinflammatory conditions:
- Determine how pathogenic inflammatory cytokine Interleukin-17 (IL-17) drives irreversible kidney damage, with the goal of revealing effective therapeutic approaches to block IL-17 signaling in chronic kidney diseases including lupus nephritis, ANCA vasculitis etc.
- To define the mechanisms of IL-17-mediated renal immunity and immunometabolic regulation of neutrophil function against invasive candidiasis, methicillin-resistant S. aureus and uropathogenic E. coli infections. The data generated from these studies will inspire the development of effective vaccines against kidney infections caused by fungal and bacterial pathogens.
- To determine the cellular and molecular mechanisms of renal fibrosis, the outcome of acute or chronic kidney diseases leading to kidney dysfunction.
- To define why patients with kidney disease are unable control infections, which is the second major cause of mortality in these patients. Using mouse models of end-stage kidney disease and dialysis patients, we systemically assess the innate, adaptive and mucosal immune dysfunction caused by uremic toxins.
|
| Publications |
Selected Publications:
- Peroumal D., Jawale C.V., Choi W., Rahimi H., Antos D., Li D.D., Wang S., Manakkat Vijay G.K., Mehta I., West R., Thangaraju M., Nolin T.D., Das J., Alcorn J.F., Biswas P.S*. (2024). The survival of B cells is compromised in kidney disease. Nature Communications. 15(1):10842. doi: 10.1038/s41467-024-55187-w. PMID: 39738044
- Li D., Jawale C.V., Zhou C., Lin L., Trevejo-Nunez G.J., Rahman S.A., Mullet S.J., Das J., Wendell S.G., Delgoffe G.M., Lionakis M.S., Gaffen S.L. and Biswas P.S*. (2022). Fungal sensing enhances neutrophil metabolic fitness by regulating antifungal Glut1 activity. Cell Host & Microbe. DOI: https://doi.org/10.1016/j.chom.2022.02.017. PMID: 35316647
Press release: https://inside.upmc.com/fighting-hospital-acquired-infections-starts-with-understanding-immune-response/
- Li D.D., Bechara R., Ramani K., Jawale C.V., Li Y., Kolls J.K., Gaffen S.L*. and Biswas P.S*. RTEC-intrinsic IL-17-driven inflammatory circuit amplifies antibody-induced glomerulonephritis and is constrained by Regnase-1. JCI Insight. PMID: 34236049
Press release:https://inside.upmc.com/gaffen-biswas-kidney-disease-research/
- Jawale C.V., Ramani K., Li D., Coleman B.M., Oberoi R.S., Kupul S., Lin l., Desai, J.V., Delgoffe, G.M., Lionakis M.S., Bender, F.H., Prokopienko A.J., Nolin, T.D., Gaffen S.L. and Biswas P.S. (2020). Restoring glucose uptake rescues neutrophil dysfunction and protects against systemic fungal infection in mouse model of kidney disease. Sci. Trans. Med. 2020 Jun 17;12(548):eaay5691. doi: 10.1126/scitranslmed.aay5691. PMID: 32554707
Highlighted in Nature Reviews Nephrology. https://www.nature.com/articles/s41581-020-032 5?proof=t%252525C2%252525A0
Featured cover art
- Jawale C.V., Li D.D., Ramani K., Lin L., Li K., Methe B. and Biswas P.S*. (2021). Uremia coupled with mucosal damage predisposes mice with kidney disease to systemic infection by commensal Candida albicans. ImmunoHorizons 15;5(1):16-24. doi: 10.4049/immunohorizons.2000114. PMID: 33451988
- Ramani K., Jawale C.V., Verma A.H., Coleman B.M., Kolls J.K. and Biswas P.S*. (2018). Unexpected kidney-restricted role for IL-17 receptor signaling in defense against systemic Candida albicans infection. JCI Insight. 2018;3(9):e98241. 10.1172/jci.insight.98241. PMID: 29720566
Highlighted in Nature Reviews Nephrology. https://www.nature.com/articles/s41581-018-0025-3.pdf
Featured Cover Art.
- Ramani K., Garg A.V., Jawale C.V., Conti H. R., Whibley N., Jackson E. K., Shiva S. S., Kolls J. K., GaffenS. L. and Biswas P.S*. (2016). The kallikrein-kinin system: a novel mediator of IL-17-driven anti-Candida immunity in the kidney. Plos Pathogens. 4;12(11):e1005952. doi: 10.1371/journal.ppat.1005952. PMID: 27814401
Recommended in F1000Prime as being of special significance in its field by F1000 Faculty Member Neil Andrew Robert Gow.
- Ramani K., Pawaria S., Maers K. Huppler A.R., Gaffen S.L. and Biswas P.S*. (2014). An essential role of interleukin-17 receptor signaling in the development of autoimmune glomerulonephritis. J. Leukoc. Biol. 96(3):463-72. PMID: 24935958
Most frequently downloaded manuscript from J. Leukoc. Biol. in first six months after publication
- Pawaria S., Ramani K., Maers K., Liu Y., Kane L., Levesque M. and Biswas P.S*. (2014). Complement component C5a permits the coexistence of pathogenic Th17 cells and type I IFN in lupus. J. Immunol. 193(7):3288-95. PMID: 25149466
Featured in the “In this Issue” section of J. Immunol. https://www.jimmunol.org/content/193/7/3179
|



