
The Facility for Experimental Radiopharmaceutical Manufacturing (FERM) is a component of the Stony Brook University PET Research Core. FERM is dedicated to the development and production of cGMP grade radiopharmaceuticals to support preclinical and clinical PET imaging at Stony Brook University.
The laboratory is directed by Professor Wenchao Qu, PhD.
The PET Research Core is directed by Professor Mark Slifstein PhD.
PET Research at Stony Brook University is directed by Professor Ramin Parsey, MD PhD, Della Pietra Family Chair of Biomedical Imaging.
Who We Are
FERM is a comprehensive core for the synthesis and characterization of PET radiopharmaceuticals. We have fully trained staff and validated equipment for the synthesis of cGMP 18F radiolabeled pharmaceuticals.
Over 500 18F radiopharmaceuticals have been characterized in vivo and over 200 of these used in humans as probes of biological systems and have applications in oncology, cardiology, neurology, psychiatry and drug development (see NIH MICAD database at http://www.ncbi.nlm.nih.gov/books/NBK5330/).
PET radiopharmaceuticals are generally administered in pharmacologically inactive amounts and are non-toxic. PET tracers are designed to be specific for a defined transport or receptor system and hence, enable the probing of basic biochemical processes in real time.
Services provided
- Full cGMP manufacturing of clinical grade radiopharmaceutical in compliance with FDA and USP guidelines
- Assistance in obtaining local (RDRC) or federal (IND) approval
- Supports both preclinical and clinical PET imaging studies
OncologyTypical oncological applications in oncology include:
|
CardiologyA number of PET tracers have been developed to target such diverse receptor systems as angiotensin-converting enzyme (ACE), endothelin receptor type A (ETA) and GLUT. Applications include: general myocardium, atherosclerosis and vascular |
|
Neurology & PsychiatryPET is the main non-invasive method for studying brain biochemistry. Oncological and cardiac PET imaging normally generate static images at equilibrium but CNS PET imaging is a dynamic process which can investigate, in real time, various biochemical processes. PET can also discriminate the effects of both chemical and behavioral stimuli on receptors and their endogenous ligands occupancy.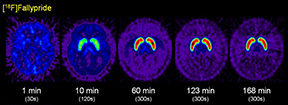 |
Drug DevelopmentPET imaging has become a cost effective tool for investigating the biodistribution of novel therapies in both preclinical and clinical phase I studies. A portion of the study drug can either be labeled with a PET isotope or a radioactive analogue created to see the efficiency of drug delivery and occupancy at a specified receptor.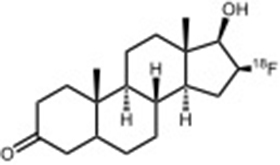 |
|

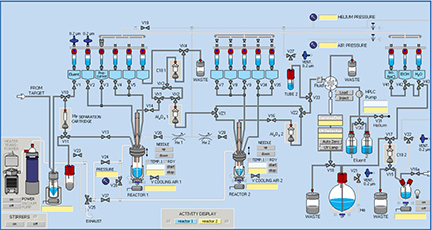
cGMP ManufacturingSynthesis Inside the hot cells are automated radiochemistry modules that perform the chemical synthesis and the final purification of the radiopharmaceutical. These radiochemistry modules are part of the triple containment system which retains all radioactivity in the facility during radiosynthesis. The units are under computer control for rapid and efficient manufacturing of consistent radiopharmaceutical products. The FERM cGMP laboratory is equipped with two MIP 1100 hot cells and a Manuela dispensing hot cell with 3" of lead shielding, two TracerLab FXN pro synthesis modules for multistep nucleophilic fluorination reactions. These modules can accommodate two pot reactions and perform the final reformulation of the radiopharmaceutical in an isotonic form for human administration. 
Synthesis Current QC equipment includes; two radio HPLC systems, a NaI(Tl) based multichannel analyzer, endotoxin tester, gamma counter, RadioTLC scanner, Gas Chromatograph |
||
|
|
||
|
Existing radiopharmaceuticals include: [18F]fallypride (dopamine D2/3) [18F]FEPPA (TSPO) [18F]VAT (vesicularacetylcholine transporter) [18F]T807 (Tau ligand for neurodegeneration) [18F]LY2459989 (κ-opioid receptor antagonist) [18F]MK-9470 (CB1R inverse agonist) [18F]Florbetaben (Amyloid ligand for neurodegeneration)
|