Intratumor heterogeneity and continuous genomic and phenotypic diversification of tumor subpopulations remain major sources of treatment failure in prostate cancer. Prostate tumors are comprised of multiple cell populations which coexist and evolve independently, diversify and compete during the course of the disease. While most current studies are focused on the evolution and the therapeutic significance of genomic alterations, less progress has been made in understanding the phenotypic evolution of cellular subpopulations and the interactions that allow them to coexist within tumors. These aspects might represent the Achilles’ heel of tumors since phenotypes are the object of natural selection in the evolutionary game of a tumor. By investigating the phenotypes of tumor subpopulations and their dynamic evolution towards clonal dominance, persistence of minor clones and castration-resistance, we are addressing fundamental questions regarding the driving forces of intratumor heterogeneity and find new evolutionary pressures that could be exploited to drive tumors towards evolutionary extinction. Moreover, we are mapping the phenotypes of dominant and minor tumor subpopulations to genomic and transcriptomic changes to provide an integrative basis for identification of high-risk clones in tumors. This will have implications for designing therapies aimed to control the evolution of drug-resistance, prevent the occurrence of advanced disease and reduce mortality. Given the staggering level of heterogeneity of human tumors, it is difficult to infer commonalities from human data. Thus, we are currently modeling intra-tumor heterogeneity and clonal evolution in mouse models and organoid culture systems for a precise mechanistic investigation of clonal properties. For this purpose, we are incorporating multicolor reporters in our studies that allows us to visualize distinct clonal populations in a different color within the same tumor. Moreover, we are utilizing computational algorithms to uncover specific genes and entire gene circuits that dictate the clonal behavior.
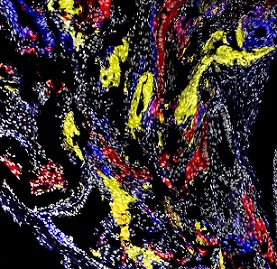
Multicolor lineage-tracing in mouse models of prostate cancer, Talos et al.
Our research will facilitate development of improved therapeutic interventions aimed to control the evolution of drug-resistant and metastatic cellular subpopulations in order to prevent the occurrence of advanced disease and reduce mortality.