Bladder cancer is a common malignancy with an estimated 430,000 new cases and 165,000 deaths annually. It has the highest lifetime treatment costs per patient of all cancers due to a high rate of disease recurrence, frequent and invasive follow-ups, and expensive techniques for diagnosis and treatment. Despite these worrying numbers, bladder cancer remains largely understudied and underfunded and little progress has been made in improving the life quality of bladder cancer patients and the overall outcome of bladder cancer treatment. In most cases of advanced disease, the treatment includes removal of the bladder wall - cystectomy - followed by reconstructive surgery - cystoplasty - usually involving colon epithelium. These interventions leave the patient with highly debilitating long-term problems such as recurrent urinary tract infections, metabolic abnormalities, and stone formation that increase costs and morbidity. Therefore, there is an urgent clinical need for alternative bladder substitutes especially in cases when native bladder cells are not suitable for tissue repair, such as advanced bladder tumors. Thus far, obtaining healthy functional autologous bladder epithelium has proved to be a challenging objective. We aim at offering a cutting-edge solution in this direction: generating de novo urinary bladder epithelium in vivo from mouse and human fibroblasts through primed transdifferentiation and tissue reprogramming. To facilitate tissue reprogramming, we are employing an unbiased systems biology approach to uncover the core regulatory transcriptional network of bladder development. This project is a clinically-relevant extension of our methodology for generating prostate epithelia from fibroblasts as described in our recent manuscript (Talos et al., Nature Communications, 2017).
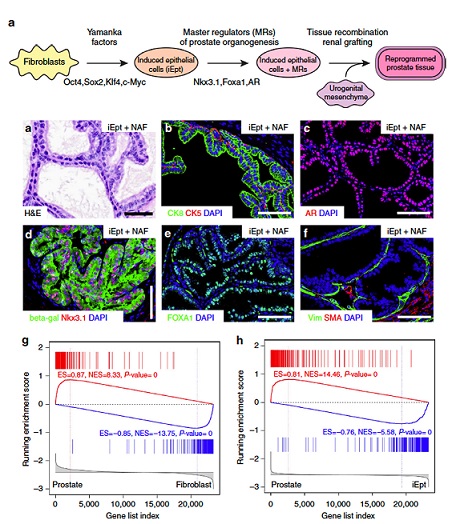
Talos et al., Nature Communications, 2017
Our studies aim at generating new ways to obtain complex tissues in vivo with a direct applicability in regenerative medicine as transdifferentiation-derived human bladder tissue could be considered for transplantation-based therapies in congenital defects (such as bladder exstrophy) or organ rehabilitation following cancer surgeries. Moreover, in combination with gene-targeting, we will use the resulting model system for mechanistic studies of bladder tumorigenesis initiation and for discovery of patient-specific early prognostic markers.