Aiming to Advance Heart Surgery for Aortic Valve Replacement

| Drs. Thomas V. Bilfinger (left) and Henry J. Tannous, our surgeons participating in the Low-Risk TAVR trial with Stony Brook Medicine interventional cardiologists. |
Aortic stenosis — narrowing of the aortic valve opening — is now the most frequently diagnosed heart valve disease. It is a potentially life-threatening condition, with a long latency period followed by rapid progression after the appearance of symptoms.
Left untreated, 50% of patients with aortic stenosis die within two years of having symptoms.
Surgical replacement of the aortic valve reduces symptoms and improves survival in patients with this illness, and in the absence of serious co-existing medical issues, the procedure is associated with very good outcomes.
However, 30% of patients with severe aortic stenosis can’t undergo the conventional valve replacement surgery, because of their advanced age and/or the presence of multiple other illnesses.
The appeal of TAVR is no surgical incision, less pain, a shorter or
no ICU stay, and faster return to normal activity.
For these high-risk patients, a less invasive treatment had been sought and, finally, the technology to achieve it was developed.
The new technology, approved by the FDA in the fall of 2011, is an aortic valve replacement device that's implanted without conventional “open heart" surgery.
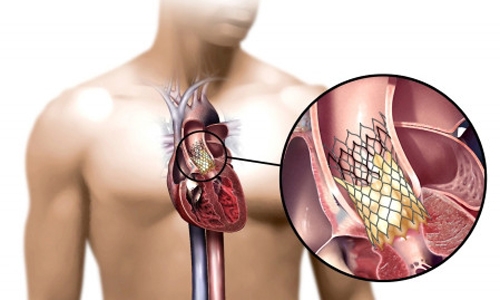
| Placement of the valve device is done from within the aorta, via catheter, without open heart surgery. |
The innovative procedure delivers the replacement valve via catheter (thin tube) while the heart is still beating. Called transcatheter aortic valve replacement (TAVR), it is performed in team fashion by cardiovascular surgeons and cardiologists working closely together.
Recovery time averages from one to two weeks. Patient selection and follow-up care involve a collaborative effort between referring physicians and our valve specialists.
Use of TAVR in patients deemed at low risk for conventional surgical valve replacement is now being studied, and in February 2017, members of Stony Brook University Heart Institute joined a multicenter clinical trial of it. Nine other select hospitals nationwide are participating.
In 2012, Stony Brook Medicine was the first in Suffolk County to offer TAVR for high-risk patients.
The co-principal investigators of the Low-Risk TAVR (LRT) trial at Stony Brook are cardiothoracic surgeon Thomas V. Bilfinger, MD, ScD, professor of surgery, and interventional cardiologist Luis Gruberg, MD, professor of medicine and director of Cardiovascular Catheterization Laboratories, who initiated the trial here.
Henry J. Tannous, MD, associate professor of surgery, of our Cardiothoracic Surgery Division is also an investigator in the study, along with four additional Stony Brook interventional cardiologists, including Puja B. Parikh, MD, MPH, assistant professor of medicine and medical director of the TAVR program.
Two different transcatheter aortic replacement valves are being used in the study at Stony Brook; namely, the CoreValve and the Sapien, as the devices are called.
| "The LRT study is the first US Food and Drug Administration-approved Investigational Device Exemption prospective multicenter feasibility trial of TAVR in low-risk patients. Patients determined to be low risk by the Heart Team will be enrolled to undergo TAVR with a commercially available balloon-expandable or self-expandable device. A propensity score-matched, site-specific cohort of historical surgical aortic valve replacement patients will serve as a control group treated during the site's enrollment period or within the prior 3 years. Enrollment commenced in 2016 and results are expected in 2018." — Feasibility of transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis, American Heart Journal (July 2017). |
Read about the TAVR program at the Stony Brook Heart Institute. For more information about the TAVR trial at Stony Brook, please call study coordinator Ruth Tenzler Stein at 631-444-3309. Watch this video (5:22 min):
