Puopolo Lab: Molecular and Cellular Mechanism of Pain
 |
Address: Email: Phone:
|
Department of Anesthesiology michelino.puopolo@stonybrookmedicine.edu 631-638-2145 |
Education
Michelino Puopolo attended the University of Bologna, Italy and received a PhD in Neurobiology and Neurophysiology from the University of Ferrara, Italy, in 1998. From 1998 to 2003 he was postdoctoral fellow in the laboratory of Dr. Elio Raviola, Department of Neurobiology, Harvard Medical School, Boston. After a brief period in Italy, in 2004 he returned to the Department of Neurobiology, Harvard Medical School, Boston, for a second postdoctoral training in the laboratory of Dr. Bruce Bean. In 2011 he joined the faculty of the Department of Anesthesiology at Stony Brook Medical Center as an Assistant Professor and was promoted to Associate Professor, with tenure in 2020. He is member of the Society for Neuroscience.
Research
My laboratory is interested in the molecular and cellular mechanism of pain. The ability to detect noxious stimuli is critical for the survival and wellbeing of an organism. As a result, individuals respond with appropriate protective behaviors to dangerous or life threatening conditions. Pain is initiated when noxious stimuli excite the peripheral terminals of small Dorsal Root Ganglia (DRG) neurons (nociceptors). Nociceptors express a variety of different ion channels (TRP channels, acid sensing ion channels, etc.) which allow these sensory neurons to transduce different types of noxious stimuli (mechanical, thermal, and chemical stimuli) into electrical signals. The initial depolarization of the cell membrane induced by a noxious stimulus leads to activation of voltage-dependent sodium channels, increase in firing rate and release of neurotransmitter (glutamate) in the dorsal spinal horn. In the setting of injuries (peripheral inflammation and/or nerve injury) ion channels expressed in nociceptors can change their level of expression or their function such that nociceptors became sensitized and hyperactive. Alterations of the pain pathway can ultimately lead to chronic, debilitating pain. As a result, innocuous stimuli (light touch or warmth) are able to induce pain (a phenomenon referred to as allodynia), or normally painful stimuli induce pain of greater intensity (a phenomenon referred to as hyperalgesia). We use electrophysiology and the patch clamp technique on acutely dissociated DRG neurons and brain/spinal cord slices to record ionic currents carried by different types of ion channels.
In addition, in collaboration with Dr. Rebecchi in our Department, we use an animal model of neuropathic pain (Chronic Constriction Injury of the sciatic nerve) to test how ion channels are affected in the setting of injuries. We have three main lines of research in the laboratory. 1) A first line of research is focusing on how inflammatory or proinflammatory molecules such as cytokines and chemokines released at the site of injury affect the functional properties of different types of ion channels expressed in nociceptors. 2) A second line of research is focusing on the pharmacology of voltage-dependent sodium channels expressed in nociceptors. In collaboration with Dr. Frank Bosmans, Johns Hopkins University, we are interested in understanding how toxins present in the venom of spiders and tarantulas interact with Tetrodotoxin-resistant (NaV1.8 and NaV1.9) sodium channels. 3) A third line of research is focusing on the descending modulation of pain. Different regions of the Central Nervous System, including noradrenergic, serotonergic, and dopaminergic nuclei, send their projections to different levels of the spinal cord. A major interest of the laboratory is to understand how different endogenous neuromodulators released by descending fibers can modulate the electrical activity of nociceptors.
A better understanding of the molecular and cellular mechanisms of acute and chronic pain will help us to identify potential therapeutic targets for the treatment of pathological conditions such as inflammatory or neuropathic pain.
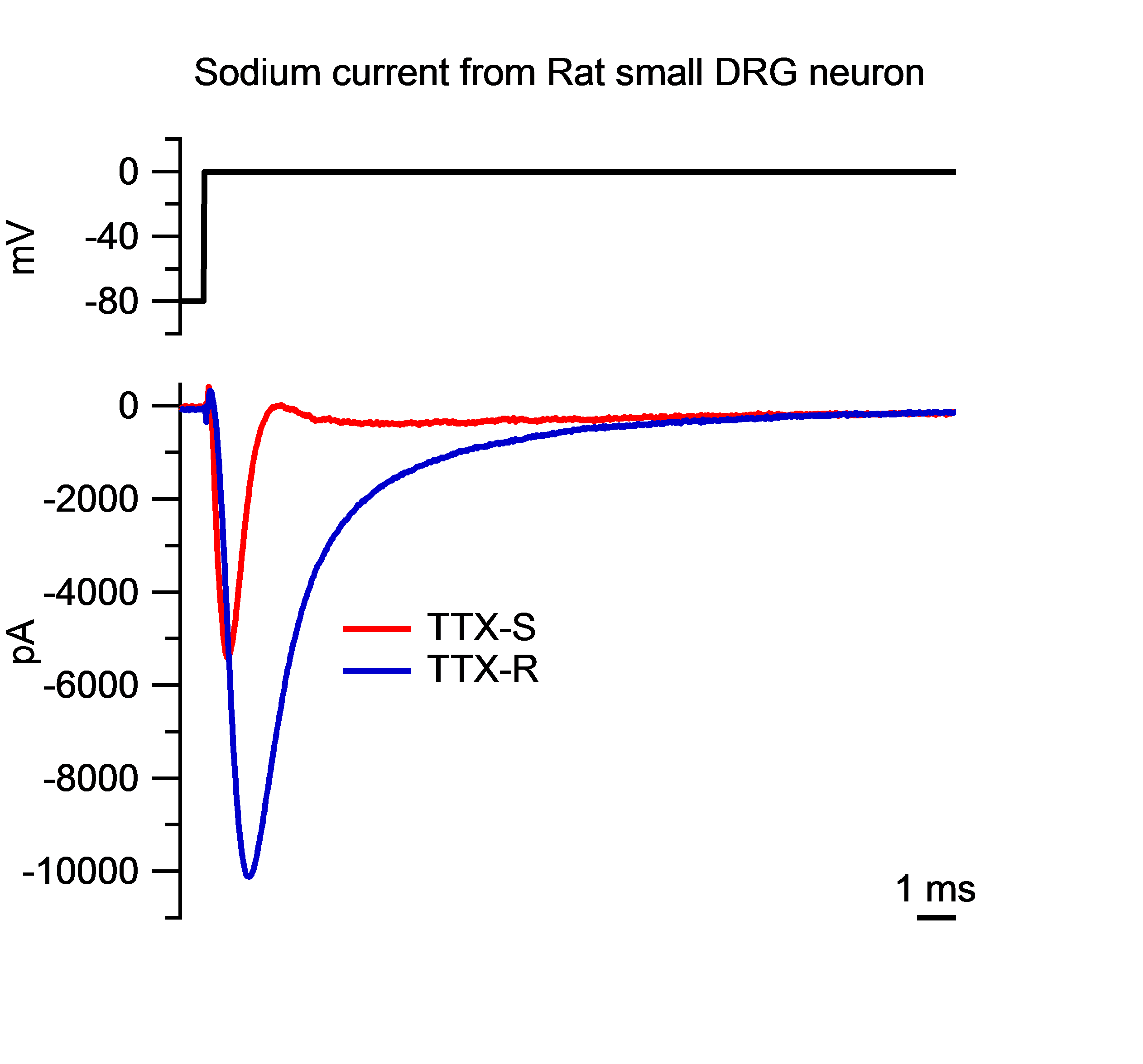
Tetrodotoxin-sensitive (TTX-S) and Tetrodotoxin-resistant (TTX-R) sodium current in nociceptor (pain-sensing) neurons. Currents were recorded from an acutely isolated small DRG neuron (<25 µm). Top: voltage-clamp protocol used to activate voltage-sensitive sodium channels. Bottom: TTX-S and TTX-R sodium currents in response to a voltage step from -80 to 0 mV. Nociceptor (pain-sensing) neurons express different isoforms of sodium channels, including TTX-S (NaV1.1, NaV1.2, NaV1.6, and NaV1.7) and TTX-R (NaV1.8 and NaV1.9). |
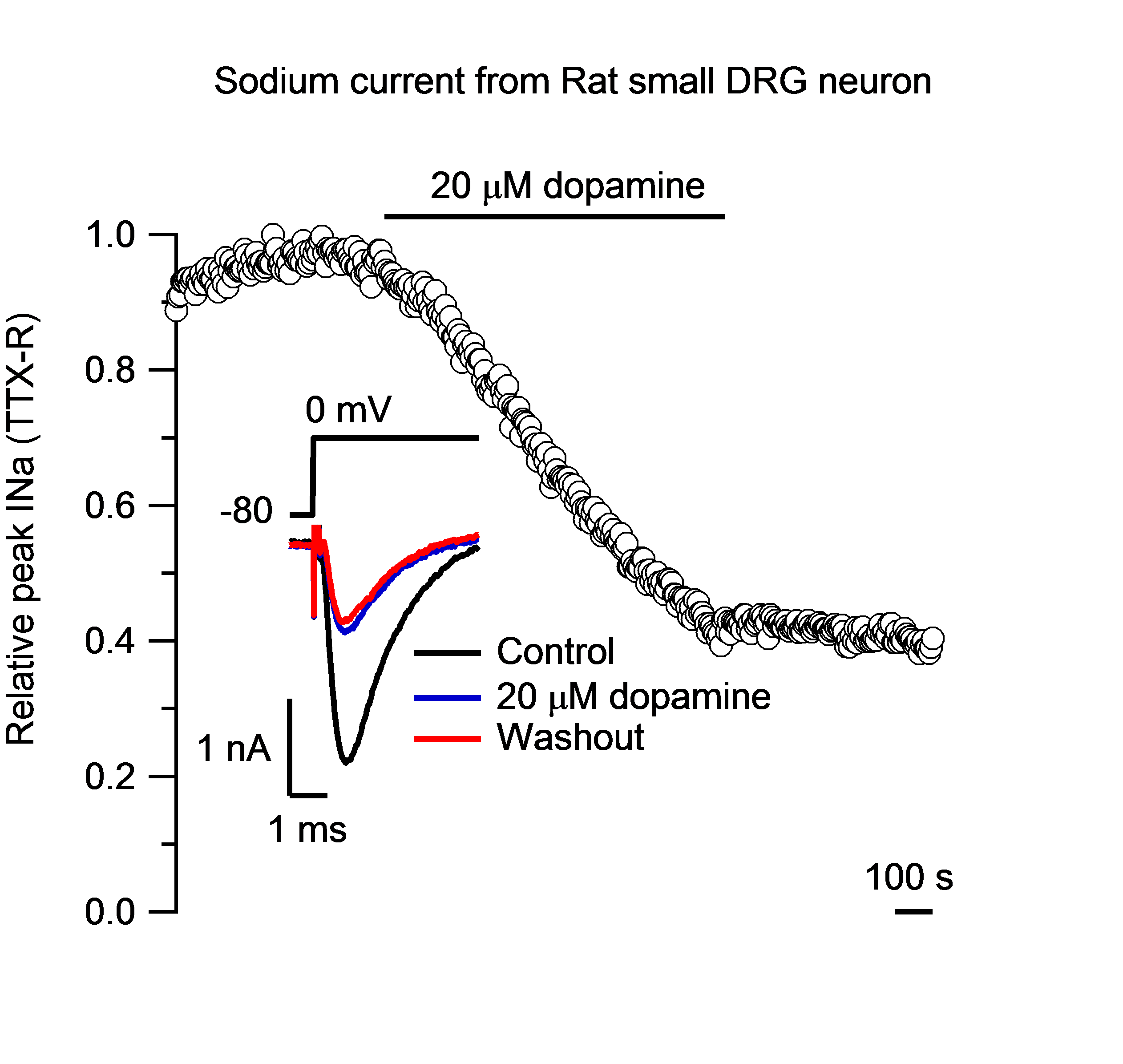
Dopamine modulation of TTX-R sodium current in nociceptor (pain-sensing) neurons. TTX-R sodium current was normalized to the peak sodium current during the first 5 min. Dopamine (20 µM) caused a reduction of the sodium current by 42%. Inset: representative sodium current traces recorded in control and after application of dopamine. |
Selected Publications
- Bosmans F, Puopolo M, Martin-Eauclaire MF, Bean BP, Swartz KJ. Functional properties and toxin pharmacology of a dorsal root ganglion sodium channel viewed through its voltage sensors. J Gen Physiol 2011 Jul;138(1):59-72.
- Binshtok AM, Gerner P, Oh SB, Puopolo M, Suzuki S, Roberson DP, Herbert T, Wang CF, Kim D, Chung G, Mitani AA, Wang GK, Bean BP, Woolf CJ. Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology 2009 Jul;111(1):127-37.
- Hirasawa H, Puopolo M, Raviola E. Extrasynaptic release of GABA by retinal dopaminergic neurons. J Neurophysiol. 2009 Jul;102(1):146-58. Epub 2009 Apr 29.
- Puopolo M, Raviola E, Bean BP. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J. Neurosci. 2007 27(3): 645-656.
- Puopolo M, Bean BP, Raviola E. Spontaneous Activity of Isolated Dopaminergic Periglomerular Cells of the Main Olfactory Bulb. J Neurophysiol. 2005 94: 3618-3627.
- Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, Raviola E. Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron 2001 30: 211-225.
- Kaczocha M, Glaser ST, Maher T, Clavin B, Hamilton J, O'Rourke J, Rebecchi M, Puopolo M, Owada Y, Thanos PK. Fatty acid binding protein deletion suppresses inflammatory pain through endocannabinoid/N-acylethanolamine-dependent mechanisms. Mol Pain. 2015 Aug 28;11(1):52
- Galbavy W, Kaczocha M, Puopolo M, Liu L, Rebecchi MJ. Neuroimmune and Neuropathic Responses of Spinal Cord and Dorsal Root Ganglia in Middle Age. PLoS One. 2015 Aug 4;10(8)
- Chakraborty S, Rebecchi M, Kaczocha M, Puopolo M. Dopamine modulation of transient receptor potential vanilloid type 1 (TRPV1) receptor in dorsal root ganglia neurons. J Physiol. 2016 Mar 15;594(6):1627-42
- Chakraborty S, Elvezio V, Kaczocha M, Rebecchi M, Puopolo M. Presynaptic inhibition of transient receptor potential vanilloid type 1 (TRPV1) receptors by norepinephrine in nociceptive neurons. J Physiol. 2017 Jan 17.
- Peng X, Studholme K, Kanjiya MP, Luk J, Bogdan D, Elmes MW, Carbonetti G, Tong S, Gary Teng YH, Rizzo RC, Li H, Deutsch DG, Ojima I, Rebecchi MJ, Puopolo M, Kaczocha M. Fatty-acid-binding protein inhibition produces analgesic effects through peripheral and central mechanisms. Mol Pain. 2017 Jan;13.
- Galbavy W, Lu Y, Kaczocha M, Puopolo M, Liu L, Rebecchi MJ. Transcriptomic evidence of a para-inflammatory state in the middle aged lumbar spinal cord. Immun Ageing. 2017 Apr 13;14:9.
- Bogdan D, Falcone J, Kanjiya MP, Park SH, Carbonetti G, Studholme K, Gomez M, Lu Y, Elmes MW, Smietalo N, Yan S, Ojima I, Puopolo M, Kaczocha M. Fatty acid binding protein 5 controls microsomal prostaglandin E synthase 1 (mPGES-1) induction during inflammation. J Biol Chem. 2018 Feb 13.
- Luk J, Lu Y, Ackermann A, Peng X, Bogdan D, Puopolo M, Komatsu DE, Tong S, Ojima I, Rebecchi MJ, Kaczocha M. Contribution of diacylglycerol lipase beta to pain after surgery. J Pain Res. 2018 Mar 5;11:473-482.
- Puopolo M. The hypothalamic-spinal dopaminergic system: a target for pain modulation. Neural Regen Res. 2019 Jun;14(6):925-930.
- Lee S, Jo S, Talbot S, Zhang HB, Kotoda M, Andrews NA, Puopolo M, Liu PW, Jacquemont T, Pascal M, Heckman LM, Jain A, Lee J, Woolf CJ, Bean BP. Novel charged sodium and calcium channel inhibitor active against neurogenic inflammation. Elife. 2019 Nov 25;8. pii: e48118.
- Lauzadis J, Liu H, Yong L, Rebecchi MJ, Kaczocha M, Puopolo M. Contribution of T-type calcium channels to spinal cord injury induced hyperexcitability of nociceptors. J Neurosci. 2020 Aug 24.
- Yang NJ, Isensee J, Neel DV, Quadros AU, Zhang HB, Lauzadis J, Liu SM, Shiers S, Belu A, Palan S, Marlin S, Maignel J, Kennedy-Curran A, Tong VS, Moayeri M, Röderer P, Nitzsche A, Lu M, Pentelute BL, Brüstle O, Tripathi V, Foster KA, Price TJ, Collier RJ, Leppla SH, Puopolo M, Bean BP, Cunha TM, Hucho T, Chiu IM. Anthrax toxins regulate pain signaling and can deliver molecular cargoes into ANTXR2+ DRG sensory neurons. Nat Neurosci. 2021 Dec 20.
- Shin SM, Lauzadis J, Itson-Zoske B, Cai Y, Fan F, Natarajan G, Kwok WM, Puopolo M, Hogan QH, Yu H. Targeting intrinsically disordered regions facilitates discovery of CaV3.2 inhibitory peptides for AAV-mediated peripheral analgesia. Pain. 2022 Apr 14.
- Bogdan DM, Studholme K, DiBua A, Gordon C, Kanjiya MP, Yu M, Puopolo M, Kaczocha M. FABP5 deletion in nociceptors augments endocannabinoid signaling and suppresses TRPV1 sensitization and inflammatory pain. Sci Rep. 2022 Jun 2;12(1):9241.
- Liu X, Bae C, Liu B, Zhang YM, Zhou X, Zhang D, Zhou C, DiBua A, Schutz L, Kaczocha M, Puopolo M, Yamaguchi TP, Chung JM, Tang SJ. Development of opioid-induced hyperalgesia depends on reactive astrocytes controlled by Wnt5a signaling. Mol Psychiatry. 2022 Oct 6.
- Liu H, Lauzadis J, Gunaratna K, Sipple E, Kaczocha M, Puopolo M. Inhibition of T-type calcium channels with TTA-P2 reduces chronic neuropathic pain following spinal cord injury in rats. J Pain. 2023 May 9